Ann Ritchie1, Mari Elisa Kuusniemi2
1Director Library & Literacy, Barwon Health, Geelong, Australia
Ann.ritchie@barwonhealth.org.au
2Science Information Specialist, Research Services, Medical Campus Library Terkko Helsinki University Library, Finland
mari.elisa.kuusniemi@helsinki.fi
Introduction
Good research data management (RDM) is an essential part of research. A data planning process ensures that all aspects of data management are holistically explored at the beginning of a research project. Short-term and long-term aims can be balanced, so that decisions that are made early in a project do not impact negatively on researchers’ ability to find and use the research data in the future.
The involvement of health librarians in RDM is becoming increasingly important in universities and hospitals around the world. Conducted during the period July-September 2015, this collaborative project drew on the expertise of a research librarian (the project leader) from a university medical library in Finland and applied this in an Australian health care library setting (the host organisation).
Objectives
The purpose of the Library RDM project was to provide the information needed for developing good RDM practices, guides and services to assist hospital-based researchers in managing their data well. The specific objectives of the project were:
- To develop library and research services at Barwon Health
- To draft a research data policy
- To create a data management planning (DMP) checklist
- To mentor Barwon Health librarians in the skills associated with managing research data.
Research design and method
The method was exploratory and consultative, using interviews and meetings with stakeholders, desktop research, role modelling and informal teaching.
The project leader and a Barwon Health librarian conducted the interviews with key informants from a range of disciplines using semi-structured interviews. Interviewees were asked about their current practices, the RDM services available to them, and what was lacking. Discussion points were:
- Data management planning advice, education and policies
- Procedures, processes and personnel
- Relevant legal and ethical issues
- Researcher workflows and practice
- The IT environment
The interviewers linked the discussion points to various topics of interest (e.g. data collection, managing active data, data documentation, data study and analysis, data publishing and/or reuse, and long term data preservation) in order to construct a comprehensive picture of RDM during the whole research lifecycle.
Desktop research was used to inform the development of policy and the DMP checklist.
Role modelling and informal teaching were the main methods used by the project leader to mentor Barwon Health Librarians.
Results
The project resulted in a clearer picture of current RDM practices at Barwon Health and a number of recommendations for improving services and research governance. The main outcomes of the project were:
- A map of the RDM lifecycle at Barwon Health, identifying available information and services and gaps in the current offering;
- A draft research data and records management policy;
- A DMP checklist.
Discussion and Conclusions
These sections compare the maturity of research data services in health libraries in the two countries and summarise the lessons learnt, with reference to the project objectives.
Keywords: Libraries, Medical; Research Support as Topic; Datasets as Topic; Information Management; Information Storage and Retrieval
Introduction
Good research data management (RDM) is an essential part of research practice. The aim of data management planning is to ensure that good scientific practice is followed, data are kept safe and secure at all stages of the research lifecycle, and that data sharing is possible after the original research has been completed.
A data planning process ensures that all aspects of data management are holistically explored at the beginning of a research project. Short-term and long-term aims can be balanced, so that decisions made early in a project do not negatively impact on the ability to find and use the research data in the future.
The following description of ‘Data Management Plan’ by the Australian National Health and Medical Research Council refers to the legal and regulatory requirements for government agencies to manage research data, and allocate resources to the provision of data (p10):
- The creation, collection, management, use and disposal of agency data is governed by a number of legislative and regulatory requirements. Government data needs to be managed in a way that ensures it is discoverable, accessible and useable. This should also include how the agency will address resource allocation in order to provide data (1).
According to the Australian Code for the Responsible Conduct of Research:
- Each institution must have a policy on the ownership of, and access to, databases and archives that is consistent with confidentiality requirements, legislation, privacy rules and other guidelines…The policy must guide researchers in the management of research data and primary materials, including storage, access, ownership and confidentiality (2).
Thus in order to comply with national requirements Barwon Health needed to develop a policy framework to assist researchers in making decisions about how to manage their data ethically and legally.
Barwon Health
Barwon Health is a large regional health service, comprising the University Hospital Geelong, and additional community and mental health, rehabilitation, aged care, and dental health services. It is located in regional Victoria, Australia, just south of Melbourne, the state’s capital. It serves a catchment population of more than 350,000 and employs more than 6,500 staff about half of whom are clinicians. The Library is primarily a clinical library, also serving educators and researchers who often have clinical appointments.
In September 2014, in line with the strategic direction of Barwon Health, the CEO established a fund to enhance and develop Barwon Health’s academic profile. The Library was awarded funds for a project to develop RDM services in the organisation as described in this article.
The following services relating to RDM existed at Barwon Health prior to commencement of the project.
The Research Ethics, Governance & Integrity (REGI) Unit provides support, advice and training to experienced and novice researchers on research design, data management and analysis, ethics applications and research dissemination. REGI manages the many aspects of research compliance, research ethics applications and review processes and gives advice and training on research design and biostatistics analysis.
Barwon Health Library provides customised support and training in conducting literature searches.
Data Warehouse collects data from several clinical and administrative systems (excluding workforce and finance systems). Reports generated from these systems are often used by researchers to identify patients suitable for enrolment in clinical trials. These reports often contain diagnosis, procedure and patient demographic details.
Senior researchers give research data management support, advice and training to novice researchers during the whole research lifecycle.
Literature review
A review of the literature shows that the involvement of health librarians in RDM is becoming increasingly important in universities and hospitals around the world.
A literature search was conducted in Ovid Medline in January 2016 using the MeSH terms Libraries, Medical (not exploded); Research Support as Topic; Datasets as Topic; Information Management; Information Storage and Retrieval. This resulted in 24 articles (1965-2016). Most articles focused on general research support services and did not specify data management as part of this service. While a number of the earlier articles from the Journal of the Medical Library Association (was Bulletin of the Medical Library Association) and the Health Information and Libraries Journal (was Health Libraries Review) foreshadowed future directions in managing research information and research data (see for example, 3-7), it has only been in recent years that health libraries have expanded their roles and responsibilities in the more specialised area of RDM.
It was therefore decided for the purposes of this literature review, to limit the search to 2001-2016. This resulted in only one suitable article which focused on data management as part of the health library’s research service (8). It was decided to supplement this article with five seminal articles about RDM services offered by academic libraries with broader responsibilities than just the health sciences disciplines. This review is therefore based on an analysis of the resulting total of five items.
Cheek and Bradigan (8) report on the results of a 2007 survey of 134 United States and Canadian academic health sciences libraries. The survey aimed to explore the libraries’ services to researchers in their organisations. They reported that the majority of libraries (more than 50%, and for some services, 100%) provided traditional research services including consultations with researchers, access to print and electronic collections, expert literature searches, workshops, and web services. Only 41.5% of libraries had librarians serving as members of a research team. RDM services were provided by the lowest numbers of libraries – data mining and data curation (12.2% each), and data base design (15.9%). Regarding education and workforce development, the authors noted: ‘CE, along with mentoring and recruiting librarians with a science background, may provide a means to further strengthen a library’s research support.’ (p 170)
Rambo (9) describes the development of RDM services at the New York University Health Sciences Library. The library conducted interviews with a sample of active researchers (11 basic scientists and 19 clinical researchers) about their needs, practices, and challenges, in order to design services to fit in with the researcher’s workflow. The findings indicated that there were issues related to collecting, organising and sharing research data. This provided the basis for the Library to develop a data catalogue (for finding and sharing datasets) and a lab organisation tool. Rambo advises other librarians who are considering implementing RDM services to consider firstly, whether their organisation has a research mission. If so, additional factors need to be taken into account: ‘the library has to have access to sufficient resources (primarily in the form of people) to dedicate to this new effort. It also has to have a degree to flexibility and autonomy to take this on.’
Tenopir, Birch, and Allard (10) conducted a survey of 351 academic libraries in the US and Canada, finding that research data services (RDS) were offered only by a minority of them. They describe the development of a new, data-intensive research environment of scientific study in which researchers often lack institutional guidance about data management. The survey found that there were significant differences in the provision of a range of RDS services offered by libraries which received NSF (National Science Foundation) grants, compared with those which received no NSF funding (p 23). The NSF requires data management plans to be submitted, and data deposition is also a funding agency requirement. They suggest that economic factors are driving the need for RDS, and this is an opportunity for libraries.
Brown, Wolski and Richardson (11) focus on skills development for the newly emerging role of research support librarian. They also situate their article in an economic framework, with an introduction about the value of research data (‘the new gold’), observing that in Australia and the United Kingdom, ‘funding bodies and national governments are seeking an improved return on investment for funded research.’ (p224)
In 2015 Briney, Gobden and Zilinski (12) reported on the library data services and institutional data policies of 206 American universities, classified as high research activity organisations. They analysed the content within these policies in terms of definitions of research data, data ownership, data retention, and conditions surrounding the separation of researchers from their institutions. The authors see opportunities for data librarians to assist researchers in navigating their organisations’ policies to meet requirements of funders and journals, as well as advocating for streamlined data sharing and reuse. ‘By leading the discussion on institutional and researcher needs, librarians can assist in creating policy as part of the overall data management infrastructure.’ They conclude that ‘the trend of library-offered data services and hiring or designation of a data librarian will become typical at major research institutions.’ (p 21)
Most recently, Barbaro (13) highlights the area of RDM as providing a unique opportunity for librarians to play an active role in the research process building on traditional skills and developing new ones. Barbaro concludes: ‘Data management is still in its emergent phase and funding agency mandates are relatively new so only a few libraries have already developed strategies to assist their researchers with their data and in creating Data Management Plans.’ (p26)
The literature review has shown that health libraries whose organisations have a research mission, and libraries in the academic sector are gradually developing their services to meet RDM requirements. To some extent this has been driven by economic factors and an opportunity to capitalise on libraries’ existing research services and infrastructure. However, RDM is a new area for librarians, and skills development is a critical need, along with resources to enable flexibility and a degree of experimentation.
Objectives
The purpose of the Barwon Health Library’s research data project was to provide the information needed for developing good research data management (RDM) practices, guides and services to assist researchers to manage their data well. This information was to be linked to the research life cycle, so that appropriate and practical guides to assist researchers in each stage of successful RDM could be developed. The project reviewed current Barwon Health RDM information, guidelines and services, and detected gaps in existing offerings.
The objectives of the project were:
- To develop library and research services at Barwon Health
- To draft a research data policy
- To create a data management planning (DMP) checklist
- To mentor Barwon Health librarians in the skills associated with managing research data.
The initial focus of the project was on drafting a research data policy and producing a data management planning (DMP) checklist to assist researchers who were in the early stages of planning a research project.
Research design and method
The project was an international collaboration, led by an experienced research librarian from Finland, Mari Elisa Kuusniemi, who was a visiting scholar at Barwon Health. Barwon Health librarians assisted in the project and were mentored by the project leader. The timeline was tight, and the whole project was carried out in 11 weeks from July to September 2015.
The method was exploratory and consultative. Data gathering methods comprised interviews with individual participants and desktop research.
Data gathering
Interviews
In the initial data gathering stage of the project, a range of stakeholders (key informants) with an interest in research at Barwon Health were invited to individual interview sessions conducted by the project leader and one of the research assistant librarians. The purpose of the interviews was to explore key informants’ research data and records management practices. Each interview was scheduled for one hour, although generally they lasted a little longer.
A semi-structured, conversational interview method was used. Interviewees were asked a set of open-ended questions to find out their perceptions and experiences of current research data management services at Barwon Health and what they believed was required.
In order to develop a comprehensive picture of the interviewees’ RDM practices and services at Barwon Health, the interview questions were framed in the context of the whole research lifecycle, comprising the following topics: data collection, managing active data, data documentation, data study and analysis, data publishing or reuse, and long term data preservation.
There are many types of research data produced in a research project. For this project, the interview questions explored the practices surrounding the following types of data produced by researchers at Barwon Health:
- clinical trial data (laboratory measurements, data from patient visits)
- qualitative data from interviews, surveys and patient records
- data about the health care system
- any clinical data where there is a need for statistical analysis
During the interviews, the interviewers used prompts to encourage the interviewees to explore the following discussion points:
- Data management planning advice, education and policies at Barwon Health
- Procedures, processes and personnel involved in conducting research
- Relevant legal and ethical issues
- Researcher workflows and practices
- The IT environment
Respondents
Responses to the interview questions were received from eighteen Barwon Health staff members (sixteen individual interviewees and two email respondents), selected on the basis of their involvement and interest in research and representing a range of departments in the organisation.
Interviews were conducted between July and September 2015 with the following categories of staff:
- four (4) with a research support role
- four (4) researchers (two of whom are also clinicians)
- four (4) who with both a research support and researcher role (one of whom is also a clinician).
Email responses to the questions were received from two participants who were not available for in-person interviews within the project’s time line.
Desktop research
An environmental scan and literature search were conducted to inform the project, specifically to identify national and international research reports, articles, and best practice examples of policy and data management planning tools.
The following categories of materials and information resources examined in this phase included:
- Shared data of RDM interviews
- Reports of RDM interviews
- Research data policies and services of Australian research organisations
- Research data planning check lists
- Research data toolkits and guides (often Libguides)
The background information found in the data gathering phase of the project was used to draft the research data and records management policy and the data management planning check list.
Mentoring
Role modelling and informal teaching were the main methods used by the Project Leader to mentor Barwon Health Librarians. The Project Leader drew up an outline of topics that could be covered, and these were to be allocated to 2 hour teaching sessions, over 11 weeks. Teaching activities included discussion, presentations, workshops, self-learning materials (specific items were suggested, including articles, reports, videos, web tools). Role modelling was implicit in all the project’s activities, as there were numerous opportunities to learn about the issues researchers faced during the course of their research and ways that librarians could help researchers to deal with and solve their RDM problems. The production of the DMP Checklist helped the Barwon Health Librarians to identify and appropriate role for the Library in supporting researchers’ work.
Results
Interview findings
This section outlines the main results of the data gathered from interviews and emails with the eighteen key informants, who were asked a set of questions structured according to the following discussion points:
- Data management planning advice, education and policies
- Procedures, processes and personnel
- Relevant legal and ethical issues
- Researcher workflows and practice
- The IT environment
The findings summarise the range of views expressed by key informants.
i. Data management planning advice, education and policies
The responses showed that the role of the Research, Ethics, Governance and Integrity (REGI) Unit is very important. Researchers receive valuable advice, guidance and support from REGI. The process of writing an ethics approval application and research protocol helps researchers to structure their research and data management plans. Researchers gave very positive feedback about the services and expertise of the REGI Unit. The research ethics applications and review processes have been accelerated, improving the research project start-up time. Other services, including research guidance and statistics support also received positive feedback.
According to the interviewees, the need for ‘some kind’ of a data management policy is clear. They noted that the policy should not be too restrictive, it should not place too many constraints on the freedom of research, and it should ensure that it is easy to follow the ‘right’ path. Creating the policy with information from reliable sources will be important but challenging, and once implemented, there will be demand for support to carry out the requirements of the data policy.
The interviewees highlighted key points that a data policy should cover and raised a number of issues needing resolution at a policy level, including:
- The need for data management plans
- Procedures for secure data storage
- Data documentation during and after a research project
- Data archiving, how and by whom?
- How documentation is to be managed at the end of a study?
- What happens when an investigator leaves?
- Who controls access to data? How the access to data should be controlled?
- Data ownership and authorship policy
- Tools recommended for data management
- Ideally a RDM policy should give an overall picture, and not be divided into multiple small pieces; the policy should be located in Prompt (Barwon Health’s document management system).
As well as needing information and guidance about how to manage research data, respondents also expressed a wish for education and training in data management skills. The main problem was viewed as the gap between theory and practice. Some training opportunities such as online courses are available, but more information and promotion of these courses is required. Having a calendar of available courses was mentioned in most of the meetings, and there was mention of an action research model linked with clinical practice that could include some RDM tasks.
In summary, the gaps in the current data management planning advice and education were:
- Lack of easy access to information needed for data management planning (there are several different channels and lack of guides)
- Training provided is not always tailored to the needs of the audience
- There are some forms which are unclear and too complicated to complete
- Need to fill the very same information in many different forms, just slightly different ways
- The education needed is sometimes available in a university, but not all Barwon Health staff members are not eligible to attend
- Lack of basic education in RDM
- Lack of education about sensitive data management
- Lack of knowledge of data sources available and expertise to use those sources (existing data from data archives)
- Lack of knowledge of available grants
ii. Procedures, processes and personnel
Currently researchers at Barwon Health need to create RDM procedures and processes by themselves. The exception is clinical trials research, where a drug company provides the guidelines for data collection and documentation, plus tools and procedures needed for good data management.
Planning RDM procedures and processes is definitely a part of research design and as one interviewee said, if there are no procedures on how to collect, document and store data ‘no one has access to the data’.
There was agreement that responsibility for managing data lies with researchers. However, respondents stated that guidelines and procedures to help researchers to conduct good RDM could be improved.
The REGI Unit helps researchers in research data governance in the planning stage and even during the research process, but the need for support is greater than the currently available resources. This is compounded by the fact that there is a lack of RDM procedures and processes and this deficiency causes an increased demand for individual support.
Interviewees were not ready for strict, regulating procedures, but enabling services and guides were desired. If possible, these should be integrated with existing procedures and RDM should not be separated from the normal research lifecycle.
The interviewees highlighted the procedures and processes, which could be helpful:
- Research data planning requirements
- Retention of research data and primary materials
- Research data record keeping
- Research data documentation (variable naming standards, etc.)
- Research data backup and reuse
- Destruction of research data
- Ownership, copyright and intellectual property (IP) of research data
- Access to research data (security, privacy, confidentiality)
- Exit planning (what happens when an investigator leaves?)
iii. Relevant legal and ethical issues
The reliability and credibility of the results of scientific research require that research complies with good scientific practice. The responsibility for abiding by good scientific practice rests with the research community as a whole and with each individual researcher. At Barwon Health, the REGI Unit’s role is to ensure that Barwon Health research complies with national and international research ethics and governance guidelines and laws and exemplifies best practice. The REGI Unit provides support, advice for ethics applications and all ethical questions during the research project.
Most of the interviewees brought out concerns about data security, privacy and confidentiality. The interviewees thought that lack of procedures and processes was one reason for this concern. Another was a lack of practical research data management tools (designed for sensitive data) or lack of knowledge of tools available.
The interviewees identified the following potential risks:
- Use of USB sticks (no encryption, easy to steal, lose or break)
- Use of Survey Monkey (especially the free version)
- Use of personal computers (no backup files, easy to steal, etc.)
- Use of external hard drives (no encryption, no backup files, easy to steal, easily broken)
- Sending files via open connections, such as email
- Storage and management of paper copies of data (surveys, interviews, patient data, etc) in open spaces (no access control system in most buildings)
- Lack of knowledge of what kind of data can be collected (qualitative research)
- Research data left behind after a researcher has left (data in network drives and paper copies in cupboards).
The ownership of data was often unclear. Most of the research projects did not make any contracts about data ownership, copyright or authorship. The interviewees did not know if they could get help from lawyers at Barwon Health for writing these kinds of contracts. Some research groups did have contract templates used in their special research area. The interviewees did not point out any cases of their own, where they had had problems due to lack of contracts. But the concern that conflict of interests could happen at some point in the future was mentioned in several meetings.
Research data left behind after a researcher had left was clearly identified as a problem. There were many cupboards full of research data and other records. The questions remained unanswered as to who takes care of archiving the data, and who can destroy the data.
iv. Researcher workflows and practice
Some of the research groups at Barwon Health had very organized workflows and practices for data management. Most often, however, the research protocol covered RDM only briefly. In addition, not all research is conducted in research groups. Often the clinical research projects are done by small groups of clinicians who don’t have much experience of research methods. They would benefit by having access to easy-to-use, practical research data management tools. The use of RDM tools would help researchers to collect, document, control access, anonymize, analyse, share, store and preserve the data.
REDCap is one such tool. REDCap (https://redcap.barwonhealth.org.au/redcap/) is a secure web-based application that supports data capture and management for research studies. It is available at no cost to Barwon Health researchers. REDCap is designed to quickly and intuitively build and manage online surveys and databases. The REGI unit provides REDCap support and training. The need for tailored education based on fundamental requirements of research groups was mentioned in the interviews. Lack of knowledge, how to benefit from the use of REDCap or how much time and resources it required to set up the database, were mentioned as the main reasons that REDCap was not used.
Currently no one has a comprehensive picture of what kinds of data there are in Barwon Health. At the beginning of a project, in the ethics application, a researcher must describe their data, but during or after the project there are no tools or practices to track this information, even though research groups and senior researchers especially, do understand the value of data collected. More generally the only way to find and reuse existing datasets is using social networks.
The following tools or infrastructure services were ‘wished for’ by the interviewees:
- Templates for contracts needed at the beginning of project
- Secure data storage with backups (safe access from offsite)
- Metadata repository or registry (a catalogue) for research data (records about the existing datasets)
- Data management planning tool or checklist
- Data archive where to preserve data after project
- Tools for data encrypting and sharing
- Tools for building a collaborative working environment
v. The IT environment
Currently the IT environment of Barwon Health is designed for clinical work, not specifically for research or education although it is also used for these purposes. There is a large conflict of needs between a highly secure, regulated and slowly changing clinical IT environment and a flexible, self-controllable and fast changing research IT environment.
All interviewees raised this issue as one of the main problems for RDM. This problem forces researchers to use their own devices and tools and this in turn creates additional problems.
There is thus a need for a parallel IT environment designed for fulfilling the requirements of research and education.
Some of the gaps of the IT infrastructure were listed as:
- Lack of storage space
- It is not easy to install the necessary software
- Lack of opportunities to tailor researchers’ own tools
- Personal profile is small (can’t save passwords, bookmarks…)
- Internet connections are slow
- Browsers are too old to use modern internet tools
- Remote access is hard to get
Project outcomes – Objective 1: Developing research and library services in the context of the Research Data Management Lifecycle
The first objective of the project was to develop research and library services at Barwon Health. The data gathering phase of the project resulted in a clearer picture of current RDM practices at Barwon Health and a number of ideas, suggestions and recommendations for improving services and research governance.
Figures 1 and 2 show the findings regarding RDM services available at Barwon Health linked to the RDM lifecycle. Figure 1 shows the existing services and Figure 2 identifies the main gaps in the current offering.
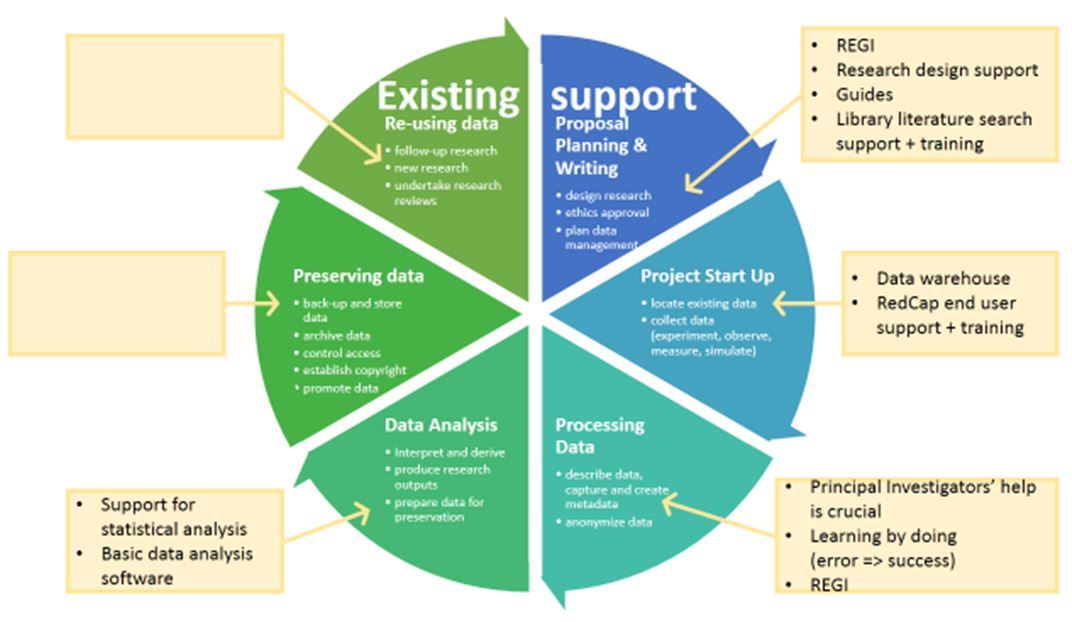
Figure 1: Existing support for researchers at Barwon Health linked to the research data management lifecycle.
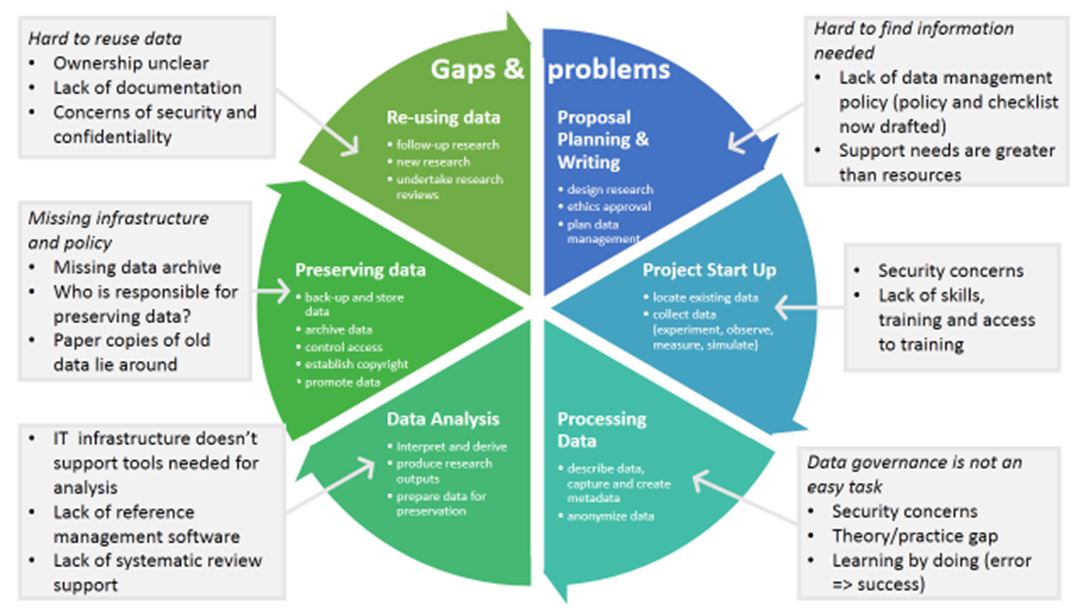
Figure 2: The main gaps in support for researchers at Barwon Health linked to the research data management lifecycle.
The project revealed valuable information on general needs and gaps of Barwon Health’s RDM support services. The data gathering stage did not cover, however, all different research areas of the hospital or all researcher groups, for example, early career researchers. The interviews were also not very detailed in some areas and not all the phases of the research lifecycle were explored in equal depth. A list of suggested interviewees for the next phase of the project has been collated, and it was recommended that additional interviews should be conducted to explore specific aspects of RDM and to improve the representativeness of the sample.
Project outcomes – Objective 2: Research Data Management (RDM) policy
The second project objective was to develop a draft RDM policy.
Desktop research provided examples and guides to developing data management policies used in several research organisations in Australia (see the website of the Australian National Data Service http://www.ands.org.au/). The meetings with researchers revealed important points which data policy should cover, as outlined above in the findings section (see a. Data management planning advice, education and policies).
The draft policy was reviewed by several interested parties, and a workshop with key stakeholders was held to further refine the policy. In late 2015 the policy was presented and endorsed by the Education, Training and Research Governance Committee and ratified by the Barwon Health Executive. Implementation of the policy will need development to support all aspects of the policy, including the development of a research data registry (with a standard set of metadata).
Project outcomes – Objective 3: Data Management Planning (DMP) checklist.
The third objective of the project was to create a data management planning tool or checklist. It was decided to create a checklist based on those used in other organisations.
After reviewing Australian and international DMP tools, the Barwon Health checklist was created. This draws particularly on the Digital Curation Centre Data Management Plan (http://www.dcc.ac.uk/resources/data-management-plans) and Arizona State University Data Management Plan Checklist (https://lib.asu.edu/data/plan) for their simplicity and ease of use.
One main finding of this project was to identify how important the ethics approval process is for RDM planning. As a part of the process researchers get support and advice. The Checklist will help researchers in their drafting of a detailed Data Management Plan for each research project, and will be integrated with the Ethics and Protocol submission process.
It was also found, however, that locating information about how to manage data was a problem for researchers as there were several sources where relevant information was housed: One Point, Prompt (the web-based repository for policies, procedures and guidelines), web pages, LibGuide, etc. The information was also located in several units or departments of Barwon Health. It was thought that one easily accessible source, where information from all these other sources is collected would be a great improvement to the current situation. The REGI Unit is currently reviewing their webpage to take this into account.
Discussion
Maturity of research data services in health libraries in the two countries
The international literature review reported in this paper has highlighted that there are various drivers for the development of RDM practices around the world, and some health libraries are capitalising on these developments. The review also found that there was a critical need for librarians to develop RDM skills, as well as having sufficient resources to enable flexibility and some willingness to experiment.
Prior to the commencement of the project, Barwon Health Library had very few services or resources aimed specifically at researchers, although the Library had implemented a digital repository for official publications (including the outputs of research, but excluding research data sets). There was no Library involvement in data management practices. Most hospital libraries in Australia would demonstrate similar levels of immaturity. In contrast, many Australian university libraries and some larger special libraries (for example, CSIRO libraries) do offer research data services, but this is not uniform across the country, and not specific to health and medical disciplines.
The situation in Finland is quite similar to Australia. There are organisations such as the University of Helsinki, where RDM services are at a more advanced stage of development, but not all organisations are at the same stage. The national guidelines for RDM, is a strength in Australia while in Finland, government is focusing on developing the national technical RDM infrastructure. A national project to implement a DMP Tool is underway, and the Barwon Health project leader has moved onto being the project manager in the Finnish initiative, with an additional role as president of one of the subgroups.
Lessons learnt
The Barwon Health project commenced at a time when a number of factors were in place for the Library to develop RDM services. There was a part-time research librarian who was new to health librarianship and would benefit from the skills development that would ensue from mentoring by the project leader. The mentoring process was also beneficial for the mentor. Due to the limited time, there was a need for rigorous prioritising. This also forced the librarians to think about the most important aspects of research librarianship. The routines, systems and the service strategy needed to be clarified and justified.
The project raised the profile of the Library within Barwon Health especially through the consultations with key stakeholders and the drafting and carriage of the RDM policy. In the area of RDM services, there are now increased expectations from clients about the possibility of support in identifying how these processes can be undertaken. The project has developed the services of both the Library and the Research Directorate, and helped to integrate RDM services more seamlessly, for example, through the introduction of the DMP Checklist as part of the Ethics and Protocol development stages.
In addition, the project has reached into the clinical education and training area. In 2016 a joint meeting of staff from the Library, Research and Clinical Education was held to design a series of workshops to cover all areas of research and academic information literacy. A training needs analysis has been designed and will be used to prioritise the courses offered in the first three months, with the intention of rolling these over to cover demand from different client groups at different times and locations.
The project was successful in achieving all of its objectives. Although some of the objectives were only partially completed, a plan was developed for the Library to carry responsibility going forward. An unintended bi-product of the project was the resignation of one of the librarians being mentored, as it was realised that being a research librarian was not the right fit for that person’s skill set and interests.
The project provided an opportunity for the project leader to focus full time on developing the library’s research services, a focus which was not possible in her normal position in the home organisation. During the project there was more time to read literature and the possibility to join seminars organised by the Australian National Data Service (ANDS). Learning about the differences between Australian and European strengths in RDM gave an excellent opportunity to increase the project leader’s RDM expertise.
Conclusion
The three-month international collaborative project gave an injection of focused activity in a specific area that would never have been achieved without tight objectives, a project framework, and the importation of specific expertise of a librarian with experience and knowledge of RDM principles and practices. The project was beneficial for all parties – the hospital’s librarians and research staff, and the visiting scholar acting as project leader.
REFERENCES
- NHMRC. 2014. Targeted Consultation Draft National Health and Medical Research Council Principles for Accessing and Using Publicly-Funded Data for Health Research Developed by NHMRC’s Prevention and Community Health Committee and the NHMRC Data Reference Group December 2014
http://consultations.nhmrc.gov.au/files/consultations/drafts/draftprinciplesaccessingpubliclyfundeddata141209.pdf - Australian Government. Australian Code for the Responsible Conduct of Research. 2007. Accessed September 2015. https://www.nhmrc.gov.au/guidelines-publications/r39
- Means ML. The Research Funding Service: a model for expanded library services. Bulletin of the Medical Library Association. 2000;88(2):178-86.
- Zink S, Illes J; Vannier MW. NLM extramural program: frequently asked questions. Bulletin of the Medical Library Association. 1996;84(2):165-81.
- Haines M. Libraries and the R&D strategy: a way forward. Health Libraries Review. 1996;13(4):193-201.
- Fletcher A. The NHS research and development programme and its impact on health care library and information resource centres. Health Libraries Review. 1995;12(4):243-7.
- Bradley J, Marshall JG. Using scientific evidence to improve information practice. Health Libraries Review. 1995;12(3):147-57.
- Cheek FM, Bradigan PS. Academic health sciences library research support. J Med Libr Assoc 2010;98(2):167-171. Doi:10.3163/1536-5050.98.2.011.
- Rambo N. Research data management: roles for libraries. Ithaka S+R. 22 October 2015. http://sr.ithaka.org?p=274643.
- Tenopir C, Birch B, Allard S. Academic libraries and research data services: current practices and plans for the future; an ACRL white paper. Chicago, IL: Association of College and Research Libraries. 2012.
- Brown RA, Wolski M, Richardson J. Developing new skills for research support librarians. The Australian Library Journal 2015;64(3):224-234. Doi:10.1080/00049670.2015.1041215.
- Briney, K, Gobden, A, Zilinski, L. Do you have an institutional research data policy? A review of the current landscape of library data services and institutional data policies. Journal of Librarianship and Scholarly Communication 2015;3(2). eP1232. http://dx.doi.org/10.7710/2162-3309.1232
- Barbaro, A. On the importance of being a data-savvy librarian. JEAHIL 2016;12(1):24-27.

